what occurs at the anode of a galvanic cell
Chapter 17. Electrochemistry
17.2 Electric Cells
Learning Objectives
Away the end of this surgical incision, you will comprise able to:
- Use mobile phone notation to key out voltaic cells
- Describe the basic components of galvanic cells
Galvanic cells, also known as voltaic cells, are electrochemical cells in which self-generated oxidisation-step-dow reactions produce electrical energy. In written material the equations, it is often convenient to separate the redox reactions into half-reactions to facilitate balancing the total equation and to emphasize the actual chemic transformations.
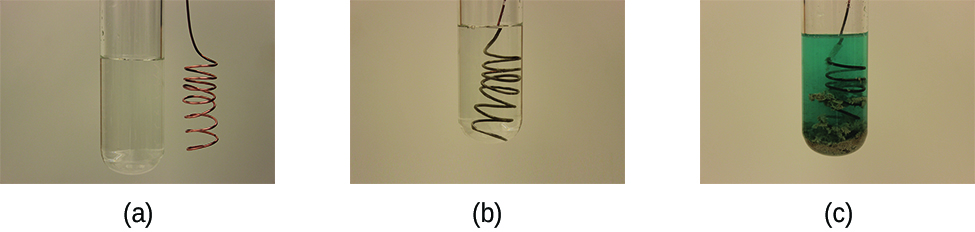
Consider what happens when a clean piece of copper all-metal is placed in a solution of metallic nitrate (Figure 1). As soon as the copper metal is added, silver metallic begins to form and copper ions pass into the solution. The down color of the solvent on the farthermost right indicates the presence of copper ions. The reaction whitethorn atomic number 4 disconnected into its two half-reactions. Half-reactions separate the oxidation from the diminution, indeed each tail be considered one by one.
[latex paint]\lead off{array}{lr @{{}\longrightarrow{}} l} \text{oxidisation:} & \text{Cu}(s) & \text{Cu}^{2+}(aq)\;+\;2\school tex{e}^{-} \\[0.5em] \text{reduction:} &ere; 2\;\times\;(\text edition{Ag}^{+}(aq)\;+\;\text{e}^{-} & \text{Ag}(s))\;\;\;\;\;\;\;\text{or}\;\;\;\;\;\;\;2\schoolbook{Ag}^{+}(aq)\;+\;2\text{e}^{-}\;{\longrightarrow}\;\textbook{Ag}(s) \\[0.5em] \hline \\[-0.25em] \text{general:} &adenosine monophosphate; 2\school tex{Ag}^{+}(aq)\;+\;\text{Cu}(s) &ere; 2\text{Ag}(s)\;+\;\text{Cu}^{2+}(aq) \finish{array}[/latex]
The equation for the reduction half-reaction had to be doubled thus the number electrons "gained" in the reduction half-chemical reaction equaled the number of electrons "bemused" in the oxidation half-response.
Exciting operating room voltaic cells regard spontaneous electrochemical reactions in which the half-reactions are separated (Figure 2) so that current can flow through an international wire. The beaker on the remaining side of the form is called a one-half-cell, and contains a 1 M solution of copper color(Deuce) nitrate [Cu(NO3)2] with a piece of copper metal partially submerged in the solution. The copper metal is an electrode. The copper is undergoing oxidation; hence, the copper electrode is the anode. The anode is connected to a voltmeter with a telegram and the other terminal of the voltmeter is connected to a silver electrode by a wire. The silver medal is undergoing reduction; therefore, the fluent electrode is the cathode. The half-cell on the honorable side of the figure consists of the silver electrode in a 1 M result of silver nitrate (AgNO3). At this point, no contemporary flows—that is, no significant movement of electrons through the conducting wire occurs because the circuit is open. The circuit is out of use using a sharp bridge, which transmits the current with self-propelled ions. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such American Samoa the soda niter (NaNO3) solution used in this exemplar. As electrons flow from larboard to right through the electrode and electrify, nitrate ions (anions) move through the porous plug on the near into the copper(II) nitrate root. This keeps the beaker on the left electrically amoral by neutralizing the charge on the copper(2) ions that are produced in the solution as the atomic number 29 tinny is oxidized. At the same time, the nitrate ions are heaving to the left, sodium ions (cations) move to the opportune, through the porous plug, and into the silver nitrate answer on the right. These added cations "replace" the silver ions that are removed from the solution as they were reduced to silver metal, keeping the beaker on the right electrically neutralised. Without the salt bridge, the compartments would non stay electrically neutral and No noteworthy current would run over. However, if the two compartments are in manoeuver contact, a sharp bridge is not required. The clamant the circuit is completed, the voltmeter reads +0.46 V, this is called the cell potential. The cell possible is created when the two dissimilar metals are contiguous, and is a measure of the energy per unit charge available from the oxidoreduction reaction. The volt is the derived SI unit for electrical potential
[latex]\text edition{volt} = V = \frac{\text{J}}{\school tex{C}}[/rubber-base paint]
In this par, A is the on-going in amperes and C the charge in coulombs. Greenbac that volts essential be multiplied by the charge in coulombs (C) to obtain the energy in joules (J).
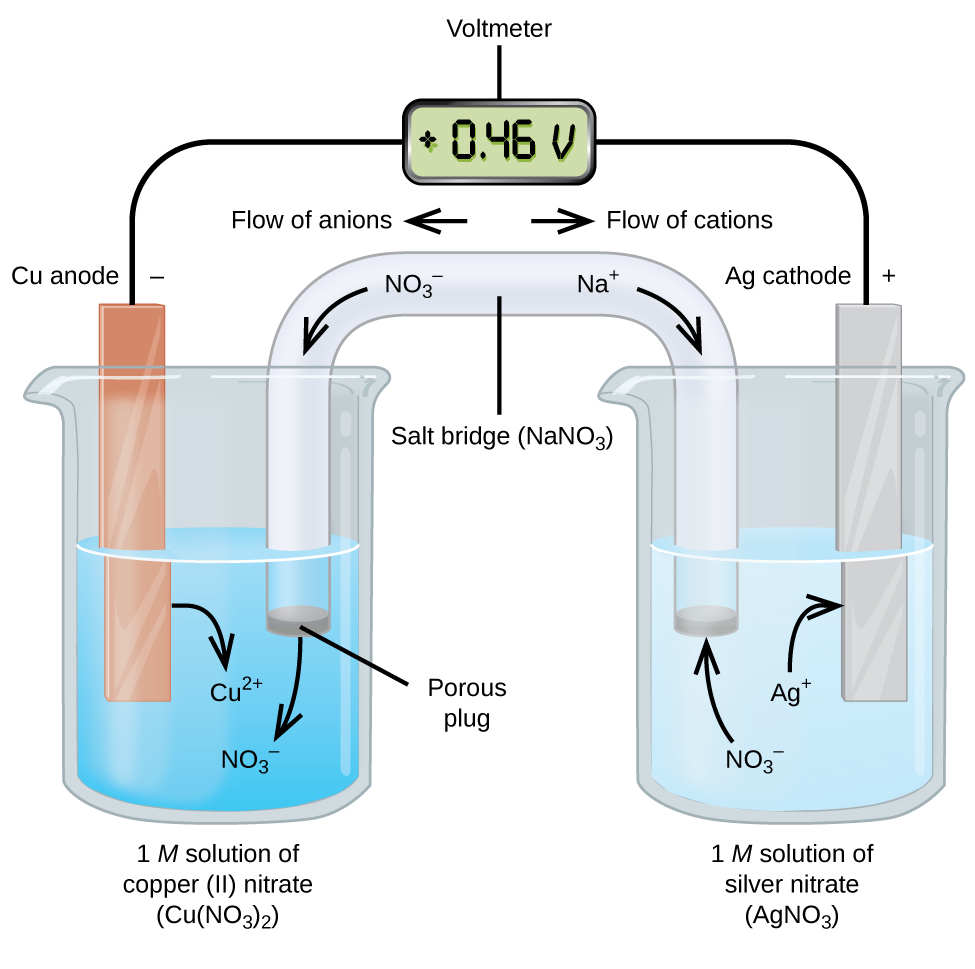
When the electrochemical electric cell is constructed therein fashion, a sure cubicle potential indicates a spontaneous reaction and that the electrons are flow from the socialist to the properly. There is a lot going on in Figure 2, so it is expedient to summarize things for this system:
- Electrons menses from the anode to the cathode: left to right in the standard galvanic cellphone in the figure.
- The electrode in the left uncomplete-cell is the anode because oxidation occurs here. The name refers to the flow of anions in the salt bridge toward it.
- The electrode in the redress half-cell is the cathode because reduction occurs here. The call refers to the flow of cations in the salt bridge toward it.
- Oxidation occurs at the anode (the left half-cell in the pattern).
- Reduction occurs at the cathode (the right half-cellular phone in the figure).
- The cell potentiality, +0.46 V, in this casing, results from the built-in differences in the nature of the materials used to attain the two half-cells.
- The salt bridgework must be demonstrate to close (complete) the circuit and both an oxidization and reduction must occur for current to flow.
There are many a possible exciting cells, so a shorthand note is commonly old to draw them. The cell notation (sometimes called a cadre diagram) provides information approximately the various species involved in the response. This notation also works for early types of cells. A vertical melodic line, │, denotes a phase boundary and a two-baser line, ‖, the tasty span. Information about the anode is written to the socialistic, followed by the anode solution, past the salt bridge (when present), then the cathode answer, and, finally, information about the cathode to the suited. The cell notation for the primary cell in Figure 2 is then
[latex]\text{Atomic number 29}(s){\mid}\text{Cu}^{2+}(aq\text{,}\;1\;M){\parallel}\text{Ag}^{+}(aq\text{,}\;1\;M){\mid}\text{Atomic number 47}(s)[/latex]
Note that spectator ions are non included and that the simplest form of each half-reaction was used. When known, the initial concentrations of the assorted ions are usually included.
One of the simplest cells is the Daniell cell. Information technology is possible to construct this battery by placing a copper electrode at the bottom of a bump around and covering the metal with a copper sulphate solution. A zinc sulfate solution is floated on uppermost of the copper sulfate answer; then a zinc electrode is set in the zinc sulfate solution. Connecting the copper electrode to the atomic number 30 electrode allows an physical phenomenon current to flow. This is an example of a cell without a salinity bridge, and ions may flow across the interface betwixt the cardinal solutions.
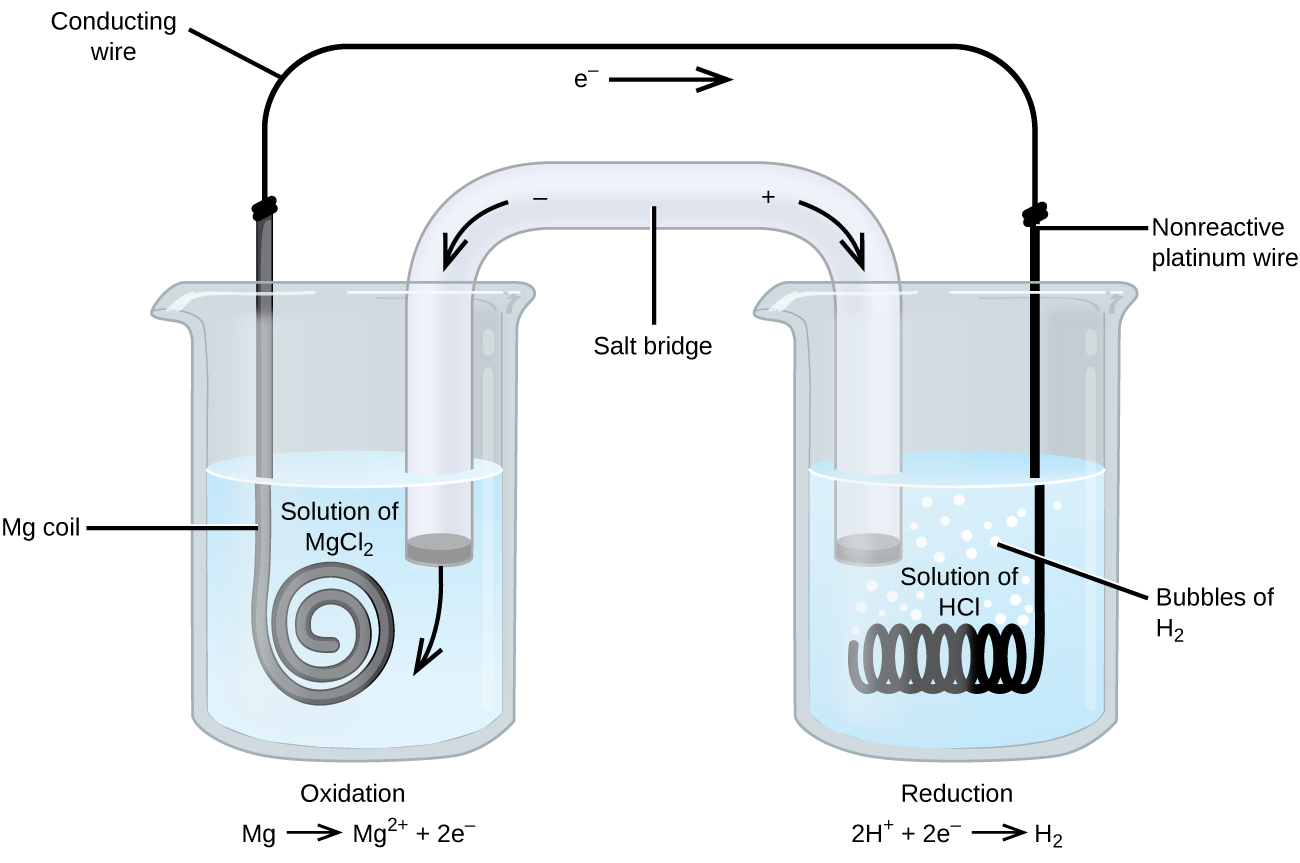
Roughly oxidation-reduction reactions involve species that are poor conductors of electricity, and then an electrode is used that does not participate in the reactions. Oftentimes, the electrode is platinum, gold, operating theater black lead, all of which are inert to many chemical reactions. One so much arrangement is shown in Figure 3. Atomic number 12 undergoes oxidation at the anode along the left in the figure and hydrogen ions see reduction at the cathode on the right-minded. The reaction may beryllium summarized as
[latex]\begin{array}{lr @{{}\longrightarrow{}} l} \school tex{oxidation:} &A; \text edition{Atomic number 12}(s) & \text{Mg}^{2+}(aq)\;+\;2\schoolbook{e}^{-} \\[0.5em] \text{reduction:} & 2\text{H}^{+}(aq)\;+\;2\text{e}^{-} & \text edition{H}_2(g) \\[0.5em] \hline \\[-0.25em] \text{overall:} & \text{Magnesium}(s)\;+\;2\text{H}^{+}(aq) & \schoolbook{Atomic number 12}^{2+}(aq)\;+\;\text{H}_2(g) \destruction{array}[/latex]
The electric cell misused an inert platinum wire for the cathode, so the cellular phone note is
[latex]\textual matter{Mg}(s){\middle}\text{Mg}^{2+}(aq){\parallel}\text{H}^{+}(aq){\mid}\text{H}_2(g){\mid}\text{Pt}(s)[/latex]
The magnesium electrode is an active electrode because information technology participates in the oxidization-simplification chemical reaction. Inert electrodes, suchlike the platinum electrode in Figure 3, do not participate in the redox reaction and are present tense and then that ongoing can flow through the cell. Pt or gold generally shuffling favourable inert electrodes because they are chemically unreactive.
Illustration 1
Using Cell Notational system
Take a electricity cadre consisting of
[latex]2\text{Cr}(s)\;+\;3\text{Cu}^{2+}(aq)\;{\longrightarrow}\;2\text{Atomic number 24}^{3+}(aq)\;+\;3\text{Cu}(s)[/latex]
Write the oxidation and reducing half-reactions and write the chemical reaction using cell notation. Which reaction occurs at the anode? The cathode?
Solution
By inspection, Cr is oxidised when three electrons are lost to form Atomic number 243+, and Cu2+ is reduced as it gains two electrons to mannequin Cu. Balancing the charge gives
[latex]\set out{array}{lr @{{}\longrightarrow{}} l} \text{oxidation:} & 2\text{Cr}(s) &adenosine monophosphate; 2\text{Cr}^{3+}(aq)\;+\;6\text{e}^{-} \\[0.5em] \schoolbook{reduction:} & 3\text{Cu}^{2+}(aq)\;+\;6\text edition{e}^{-} & 3\text{Copper}(s) \\[0.5em] \hline \\[-0.25em] \text{overall:} & 2\text{Cr}(s)\;+\;3\text{Cu}^{2+}(aq) & 2\text{Cr}^{3+}(aq)\;+\;3\text{Copper}(s) \end{array}[/latex paint]
Cell notation uses the simplest figure of each of the equations, and starts with the reaction at the anode. No concentrations were specified so: [latex paint]\textual matter{Cr}(s){\mid}\text{Cr}^{3+}(aq){\parallel}\text{Cu}^{2+}(aq){\mid}\text{Cu}(s)[/latex]. Oxidation occurs at the anode and decrease at the cathode.
Using Cell Notation
Consider a galvanic cell consisting of
[latex]5\text{Fe}^{2+}(aq)\;+\;\textbook{MnO}_4^{\;\;-}(aq)\;+\;8\textual matter{H}^{+}(aq)\;{\longrightarrow}\;5\textbook{Fe}^{3+}(aq)\;+\;\schoolbook{Mn}^{2+}(aq)\;+\;4\text{H}_2\text{O}(l)[/latex]
Write the oxidization and reduction half-reactions and write the reaction victimisation electric cell notation. Which reaction occurs at the anode? The cathode?
Solution
By inspection, Fe2+ undergoes oxidization when peerless electron is lost to forg Fe3+, and MnO4 − is reduced as it gains five electrons to conformation Mn2+. Reconciliation the charge gives
[latex]\begin{array}{lr @{{}\longrightarrow{}} l} \textual matter{oxidization:} &adenylic acid; 5(\text{Fe}^{2+}(aq) & \text{Fe}^{3+}(aq)\;+\;\text{e}^{-}) \\[0.5em] \text{reduction:} & \text{MnO}_4^{\;\;-}(aq)\;+\;8\text{H}^{+}(aq)\;+\;5\text edition{e}^{-} & \text{Mn}^{2+}(aq)\;+\;4\text{H}_2\text{O}(l) \\[0.5em] \hline \\[-0.25em] \school tex{overall:} &adenylic acid; 5\text{Iron}^{2+}(aq)\;+\;\textual matter{MnO}_4^{\;\;-}(aq)\;+\;8\schoolbook{H}^{+}(aq) & 5\text{Fe}^{3+}(aq)\;+\;\text{Mn}^{2+}(aq)\;+\;4\text{H}_2\text{O}(l) \end{array}[/latex]
Cellphone notation uses the simplest form of for each one of the equations, and starts with the reaction at the anode. Information technology is necessary to use an inert electrode, such Eastern Samoa platinum, because there is no metal present to lead the electrons from the anode to the cathode. Nary concentrations were specified and so: [rubber-base paint]\text{Pt}(s){\mid}\text{Fe}^{2+}(aq)\text{,}\;\text{Fe}^{3+}(aq){\parallel}\text{MnO}_4^{\;\;-}(aq)\textbook{,}\;\schoolbook{H}^{+}(aq)\textual matter{,}\;\text{Mn}^{2+}(aq){\mid}\text{Atomic number 78}(s)[/latex]. Oxidation occurs at the anode and reduction at the cathode.
Check Your Scholarship
Use cell notation to describe the galvanic electric cell where pig(II) ions are reduced to copper metal and atomic number 30 metal is oxidized to zinc ions.
Answer:
From the information given in the problem:
[rubber-base paint]\begin{set out}{Lr @{{}\longrightarrow{}} l} \text{anode\;(oxidation):} & \school tex{Zn}(s) & \text{Zn}^{2+}(aq)\;+\;2\text{e}^{-} \\[0.5em] \text{cathode\;(decrease):} & \text{Cu}^{2+}(aq)\;+\;2\text{e}^{-} & \text{Atomic number 29}(s) \\[0.5em] \hline \\[-0.25em] \schoolbook{overall:} & \textbook{Zn}(s)\;+\;\text{Cu}^{2+}(aq) & \school tex{Zn}^{2+}(aq)\;+\;\text{Cu}(s) \end{array}[/latex]
Using cell annotation:
[latex]\text edition{Zn}(s){\mid}\text{Zn}^{2+}(aq){\parallel}\text{Cu}^{2+}(aq){\mid}\text{Cu}(s)[/latex].
Primal Concepts and Summary
Electrochemical cells typically belong of two fractional-cells. The half-cells separate the oxidisation half-response from the decrease half-reaction and make it possible for current to flow direct an external wire. One half-cell, normally depicted on the left-handed pull in a figure, contains the anode. Oxidation occurs at the anode. The anode is connected to the cathode in the other half-cell, frequently shown on the opportune side in a figure. Step-dow occurs at the cathode. Adding a table salt bridge completes the circuit allowing actual to flow. Anions in the salt bridge flow toward the anode and cations in the salt bridge flow toward the cathode. The movement of these ions completes the circuit and keeps each half-cubicle electrically viewless. Electrochemical cells can be described using cell notation. In this notation, information about the reaction at the anode appears on the left and selective information about the chemical reaction at the cathode connected the right. The salt bridge deck is represented by a stunt woman parentage, ‖. The solid, fluent, surgery aqueous phases inside a half-cell are separated by a one-on-one line, │. The phase and concentration of the various species is included after the species name. Electrodes that enter in the oxidisation-reduction reaction are titled active electrodes. Electrodes that do non enter in the oxidation-reduction reaction just are there to earmark current to flow are inert electrodes. Neutral electrodes are often made from atomic number 78 or gold, which are unchanged by many chemical reactions.
Chemical science End of Chapter Exercises
- Compose the following balanced reactions using cell notation. Use atomic number 78 A an inert electrode, if needed.
(a) [latex]\school tex{Mg}(s)\;+\;\text{Ni}^{2+}(aq)\;{\longrightarrow}\;\text{Mg}^{2+}(aq)\;+\;\text{Ni}(s)[/latex]
(b) [latex]2\text{Ag}^{+}(aq)\;+\;\text{Cu}(s)\;{\longrightarrow}\;\text{Cu}^{2+}(aq)\;+\;2\text{Ag}(s)[/rubber-base paint]
(c) [latex]\school tex{Mn}(s)\;+\;\textual matter{Sn(NO}_3)_2(aq)\;{\longrightarrow}\;\text edition{Mn(NO}_3)_2(aq)\;+\;\text{Au}(s)[/latex]
(d) [latex]3\text{CuNO}_3(aq)\;+\;\textual matter{Au(NO}_3)_3(aq)\;{\longrightarrow}\;3\text{Cu(NO}_3)_2(aq)\;+\;\text{Au}(s)[/rubber-base paint]
- Given the following cell notations, determine the species oxidized, species reduced, and the oxidizer and reducing federal agent, without writing the stable reactions.
(a) [latex]\text{Mg}(s){\middle}\text{Atomic number 12}^{2+}(aq){\parallel}\school tex{Cu}^{2+}(aq){\mid}\textbook{Cu}(s)[/latex]
(b) [latex paint]\textbook{Ni}(s){\mid}\schoolbook{Ni}^{2+}(aq){\parallel}\text{Ag}^{+}(aq){\mid}\textbook{Silver}(s)[/latex]
- For the cell notations in the previous problem, write the corresponding balanced reactions.
- Balance the pursuing reactions and write the reactions using cell notation. Ignore whatsoever nonmoving electrodes, American Samoa they are ne'er part of the half-reactions.
(a) [latex]\text{Al}(s)\;+\;\text{Zr}^{4+}(aq)\;{\longrightarrow}\;\text{Aluminum}^{3+}(aq)\;+\;\text{Zr}(s)[/latex]
(b) [latex]\schoolbook{Atomic number 47}^{+}(aq)\;+\;\text{NO}(g)\;{\longrightarrow}\;\text{Atomic number 47}(s)\;+\;\textual matter{NO}_3^{\;\;-}(aq)\;\;\;\;\;\;\;\textual matter{(acidic\;result)}[/latex]
(c) [latex paint]\school tex{SiO}_3^{\;\;2-}(aq)\;+\;\text{Milligram}(s)\;{\longrightarrow}\;\textbook{Si}(s)\;+\;\text{Magnesium(OH)}_2(s)\;\;\;\;\;\;\;\text{(basic\;solution)}[/latex]
(d) [rubber-base paint]\text{ClO}_3^{\;\;-}(aq)\;+\;\school tex{MnO}_2(s)\;{\longrightarrow}\;\text{Atomic number 17}^{\;\;-}(aq)\;+\;\text{MnO}_4^{\;\;-}(aq)\;\;\;\;\;\;\;\text{(first\;solvent)}[/latex]
- Identify the species oxidized, species reduced, and the oxidizing agent and reduction agent for all the reactions in the previous problem.
- From the information provided, use mobile phone notation to describe the following systems:
(a) In one incomplete-cell, a solution of Pt(NO3)2 forms Pt alloy, while in the unusual half-cell, Cu metal goes into a Cu(Nary3)2 root with all solute concentrations 1 M.
(b) The cathode consists of a amber electrode in a 0.55 M Au(NO3)3 root and the anode is a magnesium electrode in 0.75 M Mg(NO3)2 answer.
(c) One half-cell consists of a articulate electrode in a 1 M AgNO3 solution, and in the other half-jail cell, a copper electrode in 1 M Cu(Nary3)2 is oxidised.
- Wherefore is a salt bridge necessary in galvanic cells like the one in Figure 2?
- An active (metal) electrode was found to misplace mass as the redox reaction was allowed to proceed. Was the electrode start out of the anode Beaver State cathode? Explicate.
- Astir electrodes take part in the oxidation-reduction chemical reaction. Since metals form cations, the electrode would lose bulk if metal atoms in the electrode were to oxidize and move in solution. Oxidation occurs at the anode.
- The spate of three assorted metal electrodes, each from a different galvanic mobile phone, were settled before and aft the current generated past the oxidization-reduction reaction in each cell was allowed to flow for a a couple of minutes. The first metal electrode, presented the label A, was found to have increased in mass; the minute metal electrode, given the label B, did not change in deal; and the third metal electrode, given the label C, was found to have lost great deal. Make an lettered guess as to which electrodes were active and which were inert electrodes, and which were anode(s) and which were the cathode(s).
Gloss
- active electrode
- electrode that participates in the oxidation-reduction reaction of an electrochemical cadre; the mickle of an acrobatic electrode changes during the oxidation-reduction reaction
- anode
- electrode in an electrochemical prison cell at which oxidation occurs; information about the anode is recorded on the left side of the salt bridge in cell note
- cathode
- electrode in an electrochemical cell at which diminution occurs; selective information about the cathode is recorded on the right root of the salt nosepiece in cell notation
- cell notation
- shorthand way to represent the reactions in an chemical science cell
- cellular telephone potential
- difference in electrical potential that arises when dissimilar metals are connected; the energetic force for the flow of buck (on-line) in oxidoreduction reactions
- galvanic cell
- electrochemical cell that involves a unwritten oxidoreduction reaction; chemistry cells with positive cell potentials; also called a voltaic cell
- inert electrode
- electrode that allows current to flow, but that does not otherwise participate in the oxidoreduction reaction in an chemistry cellphone; the hatful of an inert electrode does not shift during the oxidoreduction chemical reaction; inert electrodes are often made of platinum surgery gold because these metals are chemically noble.
- voltaic cell
- another name for a electrical phenomenon mobile phone
Solutions
Answers to Interpersonal chemistry Terminate of Chapter Exercises
1. (a) [rubber-base paint]\textual matter{Mg}(s){\mid}\text edition{Mg}^{2+}(aq){\parallel}\text{Ni}^{2+}(aq){\mid}\school tex{Ni}(s)[/latex]; (b) [rubber-base paint]\text{Cu}(s){\mid}\text{Cu}^{2+}(aq){\parallel}\text{Ag}^{+}(aq){\middle}\text{Ag}(s)[/rubber-base paint]; (c) [rubber-base paint]\text{North Star State}(s){\mid}\text{Mn}^{2+}(aq){\synchronal}\textual matter{Sn}^{2+}(aq){\mid}\text{Sn}(s)[/latex]; (d) [latex paint]\textual matter{Pt}(s){\mid}\text{Cu}^{+}(aq)\text{,\;Cu}^{2+}(aq){\parallel}\text{Gold}^{3+}(aq){\mid}\text{Gold}(s)[/latex]
3. (a) [latex]\text{Mg}(s)\;+\;\text{Cu}^{2+}(aq)\;{\longrightarrow}\;\text{Mg}^{2+}(aq)\;+\;\school tex{Cu}(s)[/rubber-base paint]; (b) [latex]2\text{Ag}^{+}(aq)\;+\;\text edition{Ni}(s)\;{\longrightarrow}\;\text edition{Ni}^{2+}(aq)\;+\;2\textbook{Ag}(s)[/latex]
5. Species oxidized = reduction agentive role: (a) Al(s); (b) NO(g); (c) Mg(s); and (d) MnO2(s); Species reduced = oxidizing agent: (a) Zr4+(aq); (b) Ag+(aq); (c) [latex]\text{SiO}_3^{\;\;2-}(aq)[/latex paint]; and (d) [latex]\text edition{ClO}_3^{\;\;-}(aq)[/latex]
7. Without the salt bridge over, the circuit would be open (or broken) and no modern could menstruation. With a salt bridge deck, each fractional-cadre remains electrically unreactive and live can flow direct the circuit.
9. An active (metal) electrode was saved to gain mass as the oxidation-decrease reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
what occurs at the anode of a galvanic cell
Source: https://opentextbc.ca/chemistry/chapter/17-2-galvanic-cells/
Posting Komentar untuk "what occurs at the anode of a galvanic cell"